


June 14, 2022 / BIOON / -- Novartis recently announced the long-term results of Eliana, a key clinical study of CD19 car-t cell therapy kymriah (tisagenlecleucel). This study is the first pediatric global car-t cell therapy registration study to evaluate the efficacy and safety of kymriah in the treatment of children with recurrent or refractory B-cell acute lymphoblastic leukemia (B-ALL) and young adults. These patients are primary refractory, chemical refractory, postoperative recurrence or do not conform to allogeneic hematopoietic stem cell transplantation (ASCT). The longest survival follow-up time was 5.9 years.
The final analysis of the study showed that 55% (95%ci:43-66) of the 79 patients treated with kymriah were still alive after 5 years; 44% of patients with remission within 3 months after infusion (n=65) were still in remission after 5 years, and the median event free survival (EFS) was 43.8 months. These findings demonstrate the long-term benefits and healing potential of kymriah. To date, kymriah is the only car-t cell therapy for these patient populations with limited previous treatment options.
The long-term follow-up of Eliana study shows that kymriah has the potential to change the cancer treatment of r/r B-ALL children and young adults, significantly improve the prognosis of this patient population, and show lasting remission and consistent safety: (1) 82% (95%ci:72-90) patients achieve remission (complete remission [cr] within 3 months after infusion or cr[cri] with incomplete hematological recovery); (2) For patients in remission, the 5-year relapse free survival (RFs) rate was 44% (95%ci:31-56), and the median RFs was 43 months; (3) No new or unexpected adverse events were reported during the long-term follow-up.
Dr. Stephan grupp, director of cancer immunotherapy cell therapy and transplantation at Philadelphia children's Hospital, said: "These data mark a promising moment for children and young people with recurrent or refractory B-cell all and their families, because recurrence of the disease is rare after 5 years. Since kymriah was approved nearly 5 years ago, we have been able to provide a real game changing option for patients who previously faced a 5-year survival rate of less than 10%."
Jeff Legos, global director and executive vice president of cancer and hematology development at Novartis, said: "At Novartis, we are committed to healing. Through the follow-up data of these treated B-cell all children and young adults for nearly 6 years, we have the strongest evidence that kymriah's one-time treatment has healing potential. These results enhance our confidence in car-t cell therapy, which is a truly transformative and paradigm shift progress in the field of cancer care."
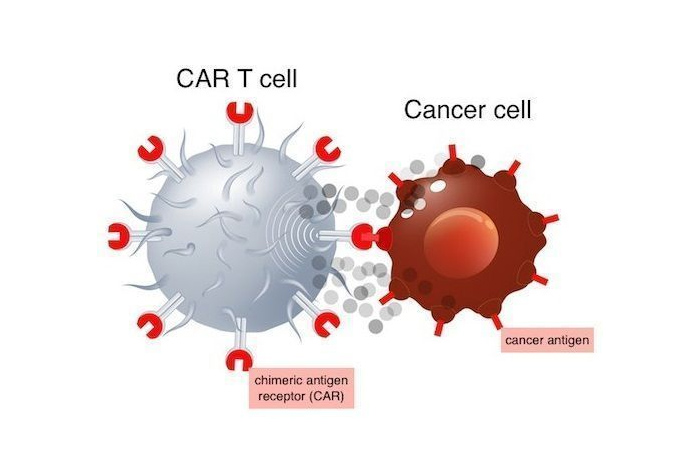
Kymriah is the first car-t cell therapy approved by FDA, which is a CD19 oriented gene modified autologous T cell immunotherapy. Unlike conventional small molecule or biological therapies, car-t cell therapy is a living T cell therapeutic product. Kymriah's principle is to genetically modify the patient's T cells to express a chimeric antigen receptor (car) aimed at targeting the antigen CD19, which is an antigen protein expressed on the surface of a variety of blood tumor cells, including B-cell lymphoma and leukemia cells.
Kymriah is a one-time treatment designed to strengthen the patient's immune system to fight cancer. Up to now, the approved indications of kymriah include: (1) treatment of children with relapsed or refractory acute lymphoblastic leukemia (r/r all) and young adults (aged to 25 years); (2) Treat adult patients with recurrent or refractory diffuse large B-cell lymphoma (r/r DLBCL); (3) Treat adult patients with recurrent or refractory follicular lymphoma (r/r FL).
